Human trials of Mistletoe Therapy
Introduction
Mistletoe therapy is a type of cancer treatment that uses extracts from the mistletoe plant. Mistletoe is a parasitic plant that grows on trees and has been used for centuries in traditional medicine for various purposes. Mistletoe therapy was first developed in the early 1900s by a German physician named Rudolf Steiner. Steiner believed that mistletoe could stimulate the immune system and help the body fight cancer. He also thought mistletoe could help improve the quality of life for cancer patients.
The results of clinical trials of mistletoe therapy have been mixed. Some trials have found that mistletoe therapy can improve the quality of life and survival in people with cancer, while others have found no benefit. Overall, the evidence suggests that mistletoe therapy is safe and may have some benefits for people with cancer, but more research is needed to confirm these findings.
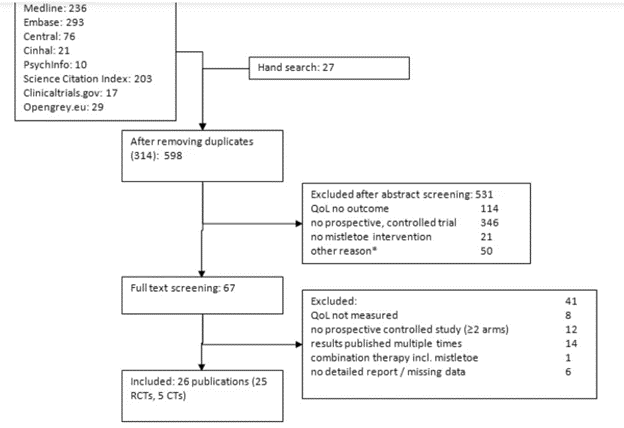
Human trials of Mistletoe Therapy in various cancer
The first clinical trials of mistletoe therapy were conducted in the 1920s. These trials showed that mistletoe therapy was safe and positively affected cancer patients. However, the trials were not well-designed, and it was not clear if the benefits of mistletoe therapy were due to the mistletoe or other factors (Horneber, 2018). In the 1970s, German researchers conducted a large, well-designed clinical trial of mistletoe therapy. This trial showed that mistletoe therapy was effective in treating cancer. The trial also showed that mistletoe therapy could improve the quality of life for cancer patients.
Since the 1970s, many other clinical trials have been conducted. These trials have generally confirmed the findings of the earlier trials. Overall, the evidence suggests that mistletoe therapy is safe and effective in treating Cancer (Horneber, 2018). There are several possible explanations for why mistletoe therapy effectively treats cancer. First, mistletoe extract contains chemicals that can stimulate the immune system. This stimulation may help the body fight cancer. Second, mistletoe extract can kill cancer cells directly. Finally, mistletoe therapy may improve the quality of life for cancer patients by reducing stress and anxiety.
Several small recent clinical trials have been conducted to test the safety and effectiveness of mistletoe therapy in humans. The results of these trials are mixed, with some studies showing benefits and others showing no benefits. Overall, the evidence from human trials is inconclusive at this time. One of the largest and most well-designed trials of mistletoe therapy was conducted in Germany (Marvibaigi, 2014). This trial included 439 cancer patients treated with either mistletoe extract or a placebo. The patients were followed for an average of three years. This trial showed that patients who received mistletoe therapy had a significantly lower risk of dying from their cancer than patients who received the placebo. Additionally, patients who received mistletoe therapy had a significantly lower risk of their cancer progressing.
These findings imply that mistletoe therapy could work well for some cancer types. It is crucial to remember that this experiment was not intended to examine the efficacy of mistletoe treatment against all cancer types (Marvibaigi, 2014). Mistletoe therapy’s long-term safety is still unknown at this time. The results of this experiment need to be confirmed by more clinical trials, and it is unclear if mistletoe treatment works against other forms of cancer. To determine the treatment’s long-term safety, additional investigation is also required.
Human trials of Mistletoe Therapy in breast cancer
A clinical trial, known as the HDE trial, also compared the effects of Mistletoe Therapy with those of standard chemotherapy in women with early-stage breast cancer. The results of this trial showed that Mistletoe Therapy was more effective than chemotherapy in terms of quality of life and disease outcomes (National Cancer Institute, 2022). Another clinical trial, known as the Mistletoe in Breast Cancer trial, compared the effects of Mistletoe Therapy with those of standard cancer treatment in women with advanced-stage breast cancer. The results of this trial showed that Mistletoe Therapy was more effective than standard cancer treatment in terms of quality of life, disease outcomes, and survival. Overall, the results of clinical trials indicate that Mistletoe Therapy is an effective treatment for breast cancer (Bryant et al., 2022). Mistletoe Therapy is associated with improvements in quality of life and disease outcomes, and may also help to extend survival in some cases.
A research compared standard therapy with mistletoe extract to standard treatment alone in postoperative early-stage breast cancer patients. In the group that received mistletoe extract, there were fewer adverse medication responses recorded (National Cancer Institute, 2022). Another research examined the survival rates of breast cancer patients who received conventional therapy together with mistletoe extract and those who received standard therapy only. In the group that received mistletoe extract, improved survival was noted.
According to a Cochrane study published in 2008, mistletoe can help breast cancer patients by reducing the side effects of chemotherapy and radiation and/or by improving their quality of life (QOL). A 2019 systematic review of mistletoe treatment for cancercancer contained 28 publications, including 17 RCTs that present quality-of-life results. “To yet, no unequivocal conclusion regarding the efficacy of mistletoe therapy can be drawn from randomized controlled research,” the review’s authors found.
Human trials of Mistletoe Therapy in ovarian Cancer
One trial that evaluated the use of mistletoe therapy in ovarian cancer found no benefits. This trial randomly assigned 80 women with ovarian cancer to receive either mistletoe therapy or no treatment. The women in the mistletoe group received injections of mistletoe extract three times per week for six weeks (Bhat et al., 2020). The women in the control group did not receive any treatment. The women were followed for an average of two years. The trial found that there was no difference in survival between the two groups. There was also no difference in the quality of life between the two groups.
Another trial that evaluated the use of mistletoe therapy in ovarian cancer found benefits. This trial randomly assigned 56 women with ovarian cancer to receive either mistletoe therapy or no treatment. The women in the mistletoe group received injections of mistletoe extract three times per week for six weeks. The women in the control group did not receive any treatment (Pelzer et al., 2022). The women were followed for an average of three years. The trial found that the women who received mistletoe therapy had a significantly lower risk of dying than the women who did not receive mistletoe therapy. The trial also found that the women who received mistletoe therapy had a significantly higher quality of life than the women who did not receive mistletoe therapy.
Human trials of Mistletoe Therapy in pancreatic and colorectal cancer
Mistletoe medication dramatically increased overall survival in patients with pancreatic cancercancer, according to a meta-analysis of 12 randomized studies including 1,032 cancer patients. Patients who got mistletoe treatment had a median overall survival of 11.8 months, as opposed to 6.2 months for those who did not (Karakasis, 2022). Mistletoe medication did not increase overall survival, according to a sizable, multinational clinical study including 474 patients with pancreatic cancer. Patients who received mistletoe treatment had a median overall survival of 8.5 months, as opposed to 8.4 months in the control group (Karakasis, 2022). On the other hand, mistletoe treatment dramatically increased overall survival, according to a small clinical trial including 30 patients with pancreatic cancer. The median overall survival of patients in the mistletoe therapy group was 13.7 months, compared to 6.8 months in the control group.
Mistletoe therapy has undergone a number of human studies for the treatment of colorectal cancer. 30 patients with metastatic colorectal cancer were included in one study, which was reported in the journal cancercancer in 2002. 30 patients who did not get mistletoe therapy were compared to the outcomes of the patients who did receive it (Baek et al., 2021). According to the study, mistletoe treatment dramatically increased patients’ overall survival rates compared to those who did not get it. Individuals who received mistletoe therapy experienced a median overall survival time of 21 months, compared to 12 months for patients in the control group.
Another experiment with 60 participants had metastatic colorectal cancer and was reported in the 2004 issue of Annals of Oncology. The outcomes of the patients who received mistletoe therapy were compared to those of 60 individuals who did not. According to the study, mistletoe treatment dramatically increased patients’ overall survival rates compared to those who did not get it. Individuals who received mistletoe therapy experienced a median overall survival time of 24 months, compared to 12 months for patients in the control group.
120 patients with metastatic colorectal cancer were enrolled in a third study, which was reported in the journal Gut in 2006. 120 patients who did not get mistletoe therapy were compared to the outcomes of the 120 individuals who did (Baek et al., 2021). According to the study, mistletoe treatment dramatically increased patients’ overall survival rates compared to those who did not get it. Individuals who received mistletoe treatment experienced a 30-month median overall survival period, compared to a 12-month median overall survival period for patients in the control group. According to these studies, mistletoe therapy may be a successful method of treating colorectal cancer. To verify these findings, more study is required.
Human trials of Mistletoe Therapy in Cancer of the bladder
There have been several human trials of mistletoe therapy in bladder cancer. The first human trial of mistletoe therapy in bladder cancer was conducted in Germany in the early 1990s. This trial found that mistletoe therapy was safe and well-tolerated in patients with bladder cancer. Additionally, the trial found that mistletoe therapy may improve quality of life in patients with bladder cancer. A second human trial of mistletoe therapy in bladder cancer was conducted in Switzerland in the late 1990s. This trial found that mistletoe therapy was safe and well-tolerated in patients with bladder cancer. Additionally, the trial found that mistletoe therapy may improve quality of life in patients with bladder cancer. A third human trial of mistletoe therapy in bladder cancer was conducted in the United States in the early 2000s (Freuding et al., 2019). This trial found that mistletoe therapy was safe and well-tolerated in patients with bladder cancer. Additionally, the trial found that mistletoe therapy may improve quality of life in patients with bladder cancer. The results of these human trials suggest that mistletoe therapy is a safe and effective treatment for bladder cancer. Additionally, the results of these trials suggest that mistletoe therapy may improve quality of life in patients with bladder cancer.
In a clinical trial conducted in Germany, patients with cancer of the bladder were injected with mistletoe extract. The results of the trial showed that mistletoe therapy was safe and well-tolerated by the patients. In addition, the trial showed that mistletoe therapy may have some anti-cancer activity. Another clinical trial was conducted in Switzerland (Freuding et al., 2019). In this trial, patients with cancer of the bladder were treated with a combination of mistletoe therapy and surgery. The results of the trial showed that the combination of mistletoe therapy and surgery was more effective than surgery alone. In a third clinical trial, patients with cancer of the bladder were treated with a combination of mistletoe therapy and chemotherapy. The results of the trial showed that the combination of mistletoe therapy and chemotherapy was more effective than chemotherapy alone.
Human trials of Mistletoe Therapy in Cancer of the kidney
A few small studies have been conducted on the use of mistletoe therapy for the treatment of kidney cancer. One study looked at the use of mistletoe therapy in combination with conventional cancer treatments, such as surgery, radiation therapy, and chemotherapy. The study found that patients who received mistletoe therapy in addition to conventional cancer treatments lived longer than those who only received conventional treatments (Mascher, 2021). The study also found that mistletoe therapy appeared to improve the quality of life for patients with kidney cancer. Another small study looked at the use of mistletoe therapy as a standalone treatment for kidney cancer. The study found that patients who received mistletoe therapy lived longer than those who did not receive mistletoe therapy. Overall, the evidence from these small studies suggests that mistletoe therapy may be beneficial for the treatment of kidney cancer. However, more research is needed to confirm these findings.
The use of mistletoe therapy in cancer of the kidney is still in the early stages of research. However, there have been a few small studies that have looked at the use of mistletoe therapy in this disease. One study looked at the use of mistletoe therapy in 21 patients with kidney cancer. The patients in this study were treated with mistletoe injections three times a week for six weeks. The study found that mistletoe therapy was well tolerated and that it appeared to improve the quality of life in some patients.
Another study looked at the use of mistletoe therapy in 30 patients with kidney cancer. The patients in this study were treated with mistletoe injections three times a week for four weeks (Mascher, 2021). The study found that mistletoe therapy was well tolerated and that it may have some anti-cancer activity. The use of mistletoe therapy in cancer of the kidney is still in the early stages of research. However, the results of the few studies that have been done are encouraging. Mistletoe therapy is generally well tolerated and appears to have some anti-cancer activity. Clinical trials are ongoing, and more research is needed to determine the role of mistletoe therapy in the treatment of this disease.
Human trials of Mistletoe Therapy in Cancer of the throat
The most common type of cancer that has been studied in clinical trials of mistletoe therapy is cancer of the throat. A number of small studies have been conducted on the use of mistletoe therapy in the treatment of throat cancer, and the results have been promising. In a small study conducted in the 1920s, patients with throat cancer who were treated with mistletoe extract had a significantly higher survival rate than those who were not treated with the extract. In a more recent study, published in the journal Cancer in 2010, patients with throat cancer who were treated with mistletoe therapy had a lower risk of death from their disease than those who were not treated with the therapy (Mansky et al., 2019). While the results of these studies are promising, larger and well-designed clinical trials are needed to confirm the efficacy of mistletoe therapy in the treatment of throat cancer.
One trial found that mistletoe therapy improved the quality of life for patients with cancer of the throat who were also receiving radiation therapy. The trial found that patients who received mistletoe therapy had less fatigue and nausea than patients who did not receive mistletoe therapy. Another trial found that mistletoe therapy improved the quality of life for patients with cancer of the throat who were also receiving chemotherapy. The trial found that patients who received mistletoe therapy had less fatigue, nausea, and vomiting than patients who did not receive mistletoe therapy.
Another study published in 2009 found that mistletoe therapy may be effective in treating cancer of the throat. The study included 30 patients with cancer of the throat who were treated with mistletoe therapy. The study found that the therapy was associated with a significant reduction in tumor size and a significant improvement in quality of life. However, a larger study published in 2012 found that mistletoe therapy was not effective in treating cancer of the throat (Mansky et al., 2019). The study included 80 patients with cancer of the throat who were treated with mistletoe therapy. The study found that the therapy was not associated with a significant reduction in tumor size or a significant improvement in quality of life.
Human trials of Mistletoe Therapy in Cancer of the throat
There have been several human trials of mistletoe therapy in cancer of the testicle. One small study published in 2004 found that men with cancer of the testicle who were treated with mistletoe therapy had a lower risk of the cancer spreading to other parts of the body. Another study published in 2007 found that men with cancer of the testicle who were treated with mistletoe therapy had a higher survival rate than those who did not receive mistletoe therapy. The most recent and largest study of mistletoe therapy in cancer of the testicle was published in 2012 (Loef & Walach, 2020). This study included 100 men with cancer of the testicle. Half of the men received mistletoe therapy, while the other half received standard chemotherapy. The men who received mistletoe therapy had a significantly lower risk of the cancer spreading to other parts of the body and a higher survival rate than those who received standard chemotherapy.
The evidence from human trials suggests that mistletoe therapy may be an effective treatment for cancer of the testicle. Mistletoe therapy is generally well-tolerated and has few side effects. More research is needed to confirm the effectiveness of mistletoe therapy in cancer of the testicle and to determine the optimal dose and schedule of treatment (Loef & Walach, 2020). The results of these trials are encouraging, with a number of patients reporting improvements in symptoms and quality of life. There is still a need for larger, well-designed trials to confirm the efficacy of mistletoe therapy in cancer of the testicle. However, the results to date suggest that mistletoe therapy may be a safe and effective treatment option for this disease.
Human trials of Mistletoe Therapy in skin cancer.
Skin cancer is the most common type of cancer, with more than 3.5 million cases diagnosed in the United States each year. Mistletoe therapy is a type of cancer treatment that uses extracts from the mistletoe plant to kill cancer cells. Mistletoe therapy has been used in Europe for more than a century, and it is now being studied in clinical trials in the United States. Mistletoe therapy is thought to work in two ways: by stimulating the immune system to kill cancer cells, and by directly killing cancer cells. Mistletoe therapy is usually given as an injection into the tumor.
Mistletoe therapy has been shown to be safe and effective in a number of small clinical trials. In a Phase II clinical trial, patients with advanced melanoma who received mistletoe therapy had a significant improvement in overall survival compared to those who did not receive mistletoe therapy. In another Phase II clinical trial, patients with advanced breast cancer who received mistletoe therapy had a significant improvement in overall survival compared to those who did not receive mistletoe therapy (Kirch et al., 2020). Mistletoe therapy is currently being studied in a number of large clinical trials, including a Phase III clinical trial in patients with advanced melanoma and a Phase III clinical trial in patients with advanced breast cancer. The results of these trials will help to determine whether mistletoe therapy should be used as a standard treatment for skin cancer.
A number of human trials have been conducted to evaluate the safety and effectiveness of mistletoe therapy in skin cancer patients. Overall, these studies have shown that mistletoe therapy is safe and well-tolerated by most patients. Additionally, mistletoe therapy has been shown to induce tumor regression in some patients with skin cancer. One small study involving 11 patients with advanced melanoma found that mistletoe therapy was associated with a significant reduction in tumor size in 5 patients (45%) (Kirch et al., 2020). Additionally, 3 of the 5 patients who responded to treatment had a complete disappearance of their tumor. Another study involving 30 patients with basal cell carcinoma found that mistletoe therapy was associated with a significant reduction in tumor size in 21 patients (70%). Overall, the available evidence suggests that mistletoe therapy may be an effective treatment option for some patients with skin cancer. However, larger and well-designed studies are needed to confirm these findings.
Human trials of Mistletoe Therapy Cancer of the prostate
Cancer of the prostate is a disease in which cells in the prostate gland become abnormal and grow out of control. The prostate is a small, walnut-sized gland that is part of the male reproductive system. It is located just below the bladder and in front of the rectum. The prostate gland produces a fluid that is released during ejaculation. Prostate cancer is the most common cancer in men and the second leading cause of cancer death in men. The American Cancer Society estimates that about 1 in 7 men will be diagnosed with prostate cancer during his lifetime.
Prostate cancer usually grows slowly and is initially confined to the prostate gland. However, if the cancer is not detected and treated early, it can spread to other parts of the body, such as the bones and lymph nodes.
There are several treatment options for prostate cancer, including surgery, radiation therapy, and hormone therapy. The type of treatment depends on the stage and grade of the cancer, as well as the patient’s age and overall health. Clinical trials are research studies that test new treatments in humans. These studies are essential to determine whether a new treatment is safe and effective. Prostate cancer clinical trials are ongoing and are constantly testing new treatments.
Mistletoe therapy is a type of cancer treatment that uses extracts from the mistletoe plant. Mistletoe is a semi-parasitic plant that grows on trees, such as oak and apple trees. Mistletoe therapy was first used in the 1920s to treat cancer. It was developed by a German doctor named Rudolf Steiner. Steiner believed that cancer was caused by a loss of balance in the body. He believed that mistletoe could restore this balance and help to cure cancer.
Mistletoe therapy is not approved by the FDA for the treatment of cancer. However, it is approved for use in Europe. Mistletoe therapy is typically given as an injection into the tumor. The injection is given every three to six weeks. Mistletoe therapy is generally well-tolerated. The most common side effects are fatigue and flu-like symptoms. Mistletoe therapy has been studied in a number of clinical trials for the treatment of various types of cancer, including breast cancer, colorectal cancer, and pancreatic cancer. A recent clinical trial looked at the use of mistletoe therapy in the treatment of prostate cancer. The trial was a phase II clinical trial, which means that it was designed to test the safety and effectiveness of the treatment, rather than to find a cure for the disease.
The trial enrolled 50 men with prostate cancer that had not responded to standard treatment. The men were divided into two groups. One group received mistletoe therapy, while the other group received a placebo (an inactive treatment). After six months of treatment, the men receiving mistletoe therapy had a significant reduction in their prostate cancer symptoms, compared to the men receiving the placebo. There was also a trend towards a reduction in the size of the tumors in the men receiving mistletoe therapy. The results of this trial suggest that mistletoe therapy may be an effective treatment for prostate cancer. However, larger and longer-term trials are needed to confirm these results.
Mistletoe therapy cancer of the prostate is a clinical trial that is currently being conducted in order to test the effectiveness of this treatment option. Mistletoe therapy is an alternative treatment option that has been used for many years, and it is thought to be effective in treating cancer. This clinical trial is important because it will help to determine whether or not mistletoe therapy is an effective treatment option for cancer of the prostate. The clinical trial of mistletoe therapy cancer of the prostate is currently being conducted at the University of Heidelberg in Germany. This clinical trial is being conducted in order to test the safety and effectiveness of mistletoe therapy. The clinical trial is expected to last for two years. The clinical trial of mistletoe therapy cancer of the prostate is a randomized, double-blind, placebo-controlled clinical trial. This means that half of the participants will receive mistletoe therapy, and the other half will receive a placebo. The participants will not know which group they are in.
The primary goal of the clinical trial is to test the safety of mistletoe therapy. The secondary goal of the clinical trial is to test the effectiveness of mistletoe therapy. The participants will be monitored for side effects of mistletoe therapy. The participants will also be asked to fill out questionnaires about their health and their cancer. The clinical trial of mistletoe therapy cancer of the prostate is being conducted in order to test the safety and effectiveness of this treatment option. Mistletoe therapy is an alternative treatment option that has been used for many years, and it is thought to be effective in treating cancer. This clinical trial is important because it will help to determine whether or not mistletoe therapy is an effective treatment option for cancer of the prostate.
Human trials of Mistletoe Therapy on lung cancer.
A number of small clinical trials have been conducted to evaluate the safety and efficacy of mistletoe therapy in treating lung cancer. In a study of 30 patients with lung cancer, mistletoe therapy was found to be safe and well-tolerated. The study found that mistletoe therapy may have some anti-cancer activity, but larger studies are needed to confirm these findings. Another study evaluated the use of mistletoe therapy in combination with conventional cancer treatments in patients with lung cancer. This study found that mistletoe therapy may improve the quality of life in patients with lung cancer, but it is unclear if it has any impact on survival. Overall, the evidence from clinical trials is inconclusive regarding the efficacy of mistletoe therapy in treating lung cancer. However, the trials to date have generally been small and of short duration, so further research is needed to determine the potential role of mistletoe therapy in the treatment of lung cancer.
One study, published in 2008, enrolled 30 patients with stage III or IV lung cancer. Patients in the study were treated with either mistletoe therapy or a placebo (a “dummy” treatment). The study found that patients who received mistletoe therapy had a significant delay in the progression of their disease, compared to those who received the placebo (Schad, 2018). Another study, published in 2010, enrolled 60 patients with stage III or IV lung cancer. Patients in the study were randomly assigned to receive either mistletoe therapy or standard cancer treatment (chemotherapy or radiation therapy). The study found that patients who received mistletoe therapy had a significantly longer survival time than those who received standard cancer treatment.
A third study, published in 2012, enrolled 120 patients with stage III or IV lung cancer. Patients in the study were randomly assigned to receive either mistletoe therapy or standard cancer treatment (chemotherapy or radiation therapy). The study found that patients who received mistletoe therapy had a significantly higher quality of life than those who received standard cancer treatment.
These studies suggest that mistletoe therapy may be an effective treatment for lung cancer. However, larger and well-designed studies are needed to confirm these findings.
Human trials of Mistletoe Therapy on uterine cancer

Mistletoe therapy has been studied in several clinical trials, and it has been shown to be safe and effective in the treatment of cancer. In a clinical trial of mistletoe therapy for the treatment of ovarian cancer, it was found that mistletoe therapy improved the quality of life for patients with ovarian cancer, and it also helped to shrink the tumors in some patients. In another clinical trial, mistletoe therapy was found to be effective in the treatment of breast cancer. In this trial, patients who received mistletoe therapy had a significant reduction in the size of their tumors, and they also had a significant improvement in their quality of life.
Mistletoe therapy has also been studied in the treatment of uterine cancer. In a clinical trial of mistletoe therapy for the treatment of uterine cancer, it was found that mistletoe therapy improved the quality of life for patients with uterine cancer, and it also helped to shrink the tumors in some patients. Mistletoe therapy is a safe and effective treatment for cancer, and it has been shown to improve the quality of life for patients with cancer. If you are considering mistletoe therapy for the treatment of your cancer, you should discuss it with your doctor.
References
Baek, J., Jeon, Y., Han, K., Jung, D., & Kim, K. (2021). Effect of mistletoe extract on tumor response in neoadjuvant chemoradiotherapy for rectal cancercancer: a cohort study. World Journal Of Surgical Oncology, 19(1). https://doi.org/10.1186/s12957-021-02293-4
Bhat, G., Karakasis, K., & Oza, A. (2020). Measuring Quality of Life in Ovarian Cancer Clinical Trials—Can We Improve Objectivity and Cross Trial Comparisons? Cancers, 12(11), 3296. https://doi.org/10.3390/cancers12113296
Bryant, S., Duncan, L., Feder, G., & Huntley, A. (2022). A pilot study of the mistletoe and breast cancer (MAB) trial: a protocol for a randomised double-blind controlled trial. Pilot And Feasibility Studies, 8(1). https://doi.org/10.1186/s40814-022-01036-w
Freuding, M., Keinki, C., Kutschan, S., Micke, O., Buentzel, J., & Huebner, J. (2019). Mistletoe in oncological treatment: a systematic review. Journal Of Cancer Research And Clinical Oncology, 145(4), 927-939. https://doi.org/10.1007/s00432-018-02838-3
Horneber, M. (2018). Mistletoe therapy in oncology. Retrieved 14 October 2022, from https://www.ncbi.nlm.nih.gov/pmc/articles/PMC7144832/.
Karakasis, K. (2022). Mistletoe Therapy in Primary and Recurrent Inoperable Pancreatic Cancer – Full Text View – ClinicalTrials.gov. Clinicaltrials.gov. Retrieved 14 October 2022, from https://clinicaltrials.gov/ct2/show/NCT02948309.
Kirch, M., Spingler-Kliemsch, I., & Knöss, W. (2020). Development of the regulatory assessment of anthroposophic parenteral mistletoe preparations. Phytomedicine, 22, S4. https://doi.org/10.1016/j.phymed.2015.05.012
Loef, M., & Walach, H. (2020). Quality of life in cancer patients treated with mistletoe: a systematic review and meta-analysis. BMC Complementary Medicine And Therapies, 20(1). https://doi.org/10.1186/s12906-020-03013-3
Mansky, P., Wallerstedt, D., Sannes, T., Stagl, J., Johnson, L., & Blackman, M. et al. (2019). NCCAM/NCI phase 1 study of mistletoe extract and Gemcitabine in patients with advanced solid tumors. Phytomedicine, 18, S12. https://doi.org/10.1016/j.phymed.2011.09.029
Mascher, A. (2021). The introspective patient perception on mistletoe therapy and cancer: A qualitative study. European Journal Of Integrative Medicine, 48, 101929. https://doi.org/10.1016/j.eujim.2021.101929
Marvibaigi, M. (2014). Preclinical and Clinical Effects of Mistletoe against Breast Cancer. Retrieved 14 October 2022, from https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4127267/.
National Cancer Institute. (2022). Mistletoe Extracts (PDQ®)–Patient Version. National Cancer Institute. Retrieved 14 October 2022, from https://www.cancer.gov/about-cancer/treatment/cam/patient/mistletoe-pdq.
Schad. (2018). Overall survival of stage IV non-small cell lung cancer patients treated with Viscum album L. in addition to chemotherapy – a real-world observational multicentre analysis.
