Role of Aerobic Glycolysis in Glucose Metabolism and Cancer Development
Cancer Development
Cancer is the result of uncontrolled aberrant cell division. In the end, some cancers might get into more tissues. As a result of quality changes, one or a few cells begin to grow and mimic erratically, which is how illness develops. A growth or tumor could develop as a result. The first place where cancer grows is called a primary tumor. Through metastasis, cancer can occasionally spread to other organs in the body. Along with blood flow, cancer and its treatments can have an impact on several physiological systems, including the immune, lymphatic, and hormonal systems. Cancer, in contrast to benign tumors, can spread throughout the body. Cancerous tumors cannot. Cancerous cells can break away from the disease’s starting point. These cells could travel throughout the body and land in lymph nodes or other organs, preventing those organs from performing their normal functions. Most cancer cells that escape is either destroyed or eliminated before they can spread to other areas. On the other hand, one or two of them may begin to move, grow, and produce new tumors. The process by which cancer spreads to a different part of the body is known as metastasis.
Glucose Metabolism
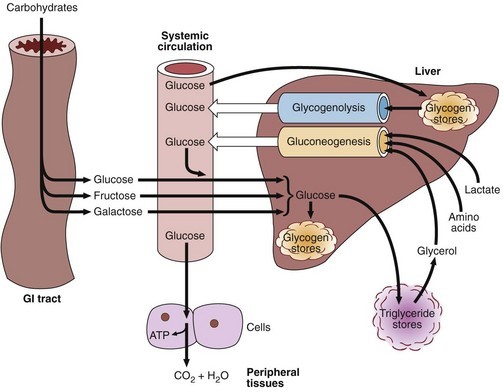
In glycogenolysis, the stored glycogen present inside the liver is broken down into glucose to meet the energy demand of the body. One of the body’s emergency responses in case of low glucose levels is gluconeogenesis in which new glucose molecules are made from raw materials such as glucogenic amino acids and glycerol. Glucose is broken by the cells in our body for energy by the process of glycolysis in which one molecule of glucose is converted into pyruvate which enters the TCA cycle in the form of acetyl CoA. Acetyl CoA can then be transformed into fatty acids through the TCA cycle, which can be used to synthesize ketone bodies (acetoacetate, -hydroxybutyrate), cholesterol, or other compounds, integrate into TAGs or oxidize them. Finally, glucose can enter the bloodstream. The liver and kidneys are the only organs that have sufficient levels of the enzyme glucose-6-phosphatase, which is needed to release glucose into the bloodstream.
Most of the important enzymes required in glucose metabolism are expressed in the liver and kidneys such as PEP carboxykinase and pyruvate carboxylase. However, the liver may also be a glycogen-producing and -absorbing organ. The kidneys participate in gluconeogenesis [1] in addition to glucose production and utilization. By strictly controlling glucose homeostasis, a person’s health and the energy requirements of their important organs are both maintained [2].
Aerobic Glycolysis, Warburg Effect and Cancer
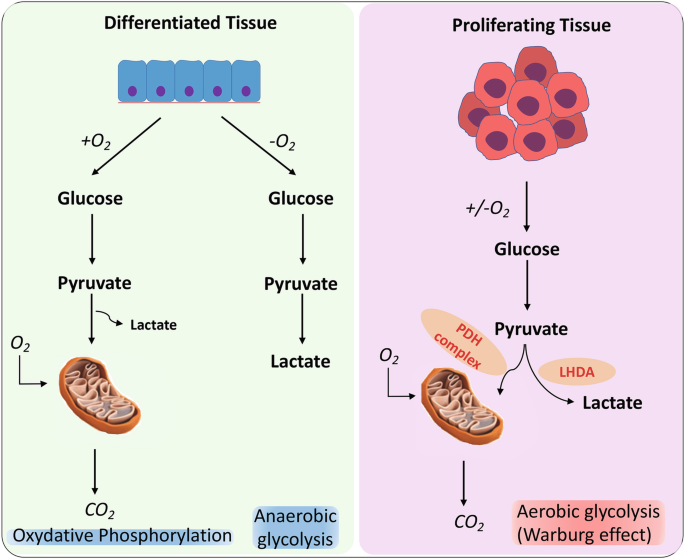
In the cytoplasm, one glucose molecule with six carbons goes through glycolysis, where it is oxidized into two pyruvate molecules with three carbons. The fate of pyruvate is determined by whether the cells contain oxygen and mitochondria. ETC is the primary location in mitochondria where oxygen is absorbed, and ATP is produced. In cells lacking mitochondria and when oxygen is absent (anaerobic conditions), glycolysis takes precedence. As lactate dehydrogenase reoxidizes NADH to NAD+, the pyruvate is debased to lactate. Erythrocytes, which need mitochondria, depend on this system as a significant wellspring of ATP. During aerobic glycolysis [3].
All tissues need glucose to function properly. Glycolysis is the primary glucose metabolism process that takes place in every cell’s cytoplasm. Depending on the oxygen available to the cells the breakdown of pyruvate can occur either by aerobic glycolysis or anaerobic glycolysis. Aerobic glycolysis occurs in two stages. In the cytosol, glucose is first converted into pyruvate, which is then used to produce NADH. This alone results in the production of two ATP molecules. The mitochondrial electron chain is reoxidized in the presence of oxygen, resulting in the release of additional free energy from NADH and 30 additional molecules of ATP for each mol of glucose.
When glucose is broken down by aerobic glycolysis then ATP is produced which is the energy source for the body This procedure ensures the survival of all mammalian life. Lactate or CO2 may be the final byproduct in animals after the glucose has been completely oxidized through mitochondrial respiration. Tumors and other cells that are proliferating or growing produce lactate and the rate of glucose absorption significantly increases even in the presence of oxygen and mitochondria that are fully functional. In-depth research has been done on the so-called Warburg Effect. ATP is the energy that is released when the carbon bonds in glucose, the primary macronutrient, are oxidized. This mechanism is necessary for the survival of every mammal. Indeed, even within the sight of oxygen and practical mitochondria, the pace of glucose assimilation ascends in growths and other multiplying or developing cells, and lactate is created. The phenomenon known as the Warburg Effect has received extensive study. Warburg Effect has been proposed as a reprogrammed mechanism for meeting the metabolic requirements of unchecked growth. In this instance, the increased intake of glucose supplies the anabolic reactions that are necessary to encourage cell growth with carbon [4]. High-impact glycolysis is utilized by numerous cells, including lymphocytes and microorganisms, when they multiply rapidly, showing that it very well might be significant for advancing cell advancement [5].
Role of Aerobic Glycolysis in Cancer Development
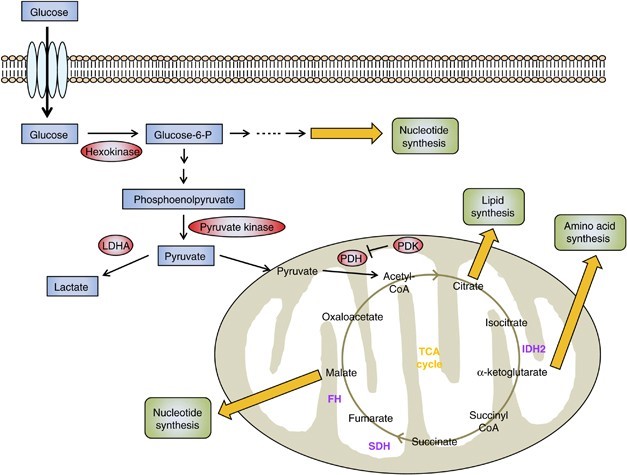
To maintain the equilibrium of cellular energy, increased glycolysis compensates for impaired oxidative phosphorylation under hypoxia. Most solid tumor cells, despite having an active oxidative phosphorylation apparatus, switch their metabolism from respiration to glycolysis, which is known as aerobic glycolysis in cancer [6]. During the lack of oxygen when oxidative phosphorylation is inhibited then lactate fermentation is activated to meet the energy demand. Because of the continuous growth required for cancer cells to divide and make nucleotide stockpiles they need a lot more energy. So, this implies why the Warburg effect is important for cancer cell survival and proliferation [7]. When we compare two processes; one in which glucose is aerobically broken down into pyruvate and then TCA cycle to yield the maximum potential of the glucose, while in anaerobic glycolysis the energy yield process is less efficient. So, cancer cells utilize aerobic glycolysis and there is more demand for glucose by cancer cells which is 100 times more than normal cells. The Warburg effect is the result of the coordination of these various processes. In changed disease cells, when glucose retention and glycolytic exercises are recognizably expanded, there happens a drawn-out metabolic reinventing that outcomes in the reliance on fermentative digestion for ATP creation.
When the respiratory system is normal, different methods will be used to control glycolytic activity to keep energy levels in balance. Many types of cancer cells require glutamine as a crucial bioenergetic and anabolic substrate, despite Warburg’s lack of emphasis on it. Apart from glucose sources for energy cancer cells also utilize glutamine in the Warburg effect. TCA cycle and other metabolic pathway intermediates are also provided by glutamate. Cancer cells to continue the TCA cycle, require glutamine, just as they do for glycolysis. Glycolysis prevents the development of tumors and the proliferation of cells. The suppression of metabolic pathways related to glycolysis also slows the growth of tumors. These points suggest that inhibiting glycolysis might be an effective way to slow or stop cancer growth.
Warburg Effect and Mutations lead to Cancer
The movement of metabolic catalysts can be emphatically influenced by changes that enact oncogenes or deactivate growth silencers, and these transformations have a critical impact on the oxygen-consuming glycolysis of disease. Oncogenic mutations that can alter cellular metabolism include modifications in PI3K, PTEN, Myc, and p53, as well as phosphatase and tensin homolog (PTEN). Since the PI3K pathway is observed to be transformed, it has a critical impact on growth endurance and multiplication in a great many human malignancies. After that, PI3K activation causes AKT to become active, stabilizing hypoxia-inducible factor (HIF)-1. The growth silencer PTEN is itself repressed by the PI3K catalyst, and PTEN misfortune upgrades glycolysis through initiating AKT and HIF-1. Together with HIF, Myc activates glycolytic enzymes, glucose transporters, lactate dehydrogenase A, and PDK1. Myc can also activate genes that are related to the biogenesis and function of mitochondria [8].
Warburg’s effect explained how cancer cells reprogrammed their metabolic machinery to their benefit to obtain maximum energy for proliferation and metastasis. But most of this reprogramming was found to be due to the mutations in the genes which are responsible for aerobic glycolysis. The most common mutations leading to cancer are fumarate hydratase and succinate dehydrogenase mutations. Genome sequencing research of cancer has found a link between carcinogenesis and a mutation in a metabolic enzyme. Isocitrate dehydrogenases IDH1 and IDH2, which are dependent on NADP (nicotinamide adenine dinucleotide phosphate), provide a link of mutations in the metabolic reprogramming of cancer. While converting isocitrate into -ketoglutarate (KG), IDHs conduct the formation of one NADPH molecule in human cells. The cytoplasm and mitochondria are where the homodimer enzymes IDH1 and IDH2 operate, respectively [9]. Cancer cells frequently exhibit activation of Akt. It has been demonstrated that a variety of human cancers cause an increase in Akt. At first, Akt was described as the cellular counterpart of a viral oncogene. The loss of phosphatidylinositide-3-kinase (PI3K), an upstream activator of Akt, or amplification of phosphatidylinositide-3-kinase (PI3K), a PI3K antagonist, are two examples of indirect mechanisms by which cancer cells acquire constitutive Akt activity. Bcr-Abl, Her2/neu, and Ras all rely on Akt as a crucial oncogene downstream effector [10].
References
- Melmed, S., Koenig, R., Rosen, C., Auchus, R., & Goldfine, A. (2020). Williams textbook of endocrinology: South Asia edition, 2 vol set-E-book. Elsevier India.
Link: https://www.sciencedirect.com/topics/medicine-and-dentistry/glucose-metabolism - Han, HS., Kang, G., Kim, J. et al. Regulation of glucose metabolism from a liver-centric perspective. Exp Mol Med 48, e218 (2016). https://doi.org/10.1038/emm.2015.122
Link: https://www.nature.com/articles/emm2015122#citeas - Dashty M. A quick look at biochemistry: carbohydrate metabolism. Clin Biochem. 2013 Oct;46(15):1339-52. doi: 10.1016/j.clinbiochem.2013.04.027. Epub 2013 May 14. PMID: 23680095.
Link: https://pubmed.ncbi.nlm.nih.gov/23680095/ - Liberti MV, Locasale JW. The Warburg Effect: How Does it Benefit Cancer Cells? Trends Biochem Sci. 2016 Mar;41(3):211-218. DOI: 10.1016/j.tibs.2015.12.001. Epub 2016 Jan 5. Erratum in: Trends Biochem Sci. 2016 Mar;41(3):287. Erratum in: Trends Biochem Sci. 2016 Mar;41(3):287. PMID: 26778478; PMCID: PMC4783224.
Link: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4783224/ - Niu X, Arthur P, Abas L, Whisson M, Guppy M. Carbohydrate metabolism in human platelets in a low glucose medium under aerobic conditions. Biochim Biophys Acta. 1996 Oct 24;1291(2):97-106. doi: 10.1016/0304-4165(96)00051-7. PMID: 8898869.
Link: https://pubmed.ncbi.nlm.nih.gov/8898869/ - Vogelstein, B., & Kinzler, K. W. (2004). Cancer genes and the pathways they control. Nature medicine, 10(8), 789-799.
Link: https://www.nature.com/articles/nm1087 - DeBerardinis, R. J., Lum, J. J., Hatzivassiliou, G., & Thompson, C. B. (2008). The biology of cancer: metabolic reprogramming fuels cell growth and proliferation. Cell metabolism, 7(1), 11-20.
Link: https://www.sciencedirect.com/science/article/pii/S1550413107002951 - Pfeiffer, T., Schuster, S., & Bonhoeffer, S. (2001). Cooperation and competition in the evolution of ATP-producing pathways. Science, 292(5516), 504-507.
Link: https://www.science.org/doi/abs/10.1126/science.1058079 - Dang, C. V., O’Donnell, K. A., Zeller, K. I., Nguyen, T., Osthus, R. C., & Li, F. (2006, August). The c-Myc target gene network. In Seminars in cancer biology (Vol. 16, No. 4, pp. 253-264). Academic Press.
Link: https://www.sciencedirect.com/science/article/pii/S1044579X06000654 - Elstrom, R. L., Bauer, D. E., Buzzai, M., Karnauskas, R., Harris, M. H., Plas, D. R., … & Thompson, C. B. (2004). Akt stimulates aerobic glycolysis in cancer cells. Cancer research, 64(11), 3892-3899.
Link: https://aacrjournals.org/cancerres/article/64/11/3892/511348/Akt-Stimulates-Aerobic-Glycolysis-in-Cancer-Cells
