HIGH-DOSE VITAMIN C SUPPRESSES VASCULAR ENDOTHELIAL GROWTH FACTOR (VEGF) EXPRESSION
Summary
Numerous studies have been carried out to look at the anti-cancer properties of vitamin C. (VitC). The studies were focused on high-dose VitC and examined the impact of high-dose VitC (4 g/kg) on vascular endothelial development in mice receiving xenografts of Colon 26 rectal cancer cell line. After establishing male mice with Colon 26 tumors, high-dose VC solution was given to them once daily for 14 days. The lower limb tumor tissues and serum samples were collected on the last day of the study, and they were examined for the expression of proteins associated with tumor angiogenesis and the concentrations of reactive oxygen species. Oral VitC administration increased p53 and endostatin levels while reducing tumor volumes. In addition, plasma and in tumor part ROS levels and tissue hypoxia inducible factor-1α (HIF-1α) were reduced by VitC administration. In addition, the levels of vascular endothelial growth factor A (VEGFA) and vascular endothelial growth factor D (VEGF D) were decreased by VitC administration. These results suggest that VitC exerts its anti-cancer effects by suppressing angiogenesis.
INTRODUCTION
Vascular endothelial growth factor (VEGF) is a major regulator of angiogenesis and is involved in solid tumor carcinogenesis, infiltration, distant metastasis, and tumor angiogenesis. Angiogenesis in tumors is crucial in regulating the development of cancer. VEGF subtypes come in various varieties, each with its own special qualities. VEGFA is the major subtype that efficiently causes the proliferation of endothelial cells and enhances their permeability. In advanced colorectal cancer, a subtype called VEGFD has a strong angiogenic effect. Under numerous circumstances linked to cancer, such as the presence of various growth factors and hypoxia, VEGF is upregulated. The transcription factor controls how hypoxia-induced transcription enhancement of the VEGF gene works. HIF-1, or hypoxia-inducing factor. The creation of new vasculature in and around a tumor is a result of the synthesis of VEGF and other growth hormones by the tumor, but the new vascular network is architecturally and functionally aberrant. Endostatin, a VEGF inhibitor that is an endogenous inhibitor of angiogenesis and tumor growth, is a C-terminal fragment of collagen XVIII. Endostatin has reportedly played a role in the partial inhibition of tumors by p53.
Vitamin C (VitC) is a water-soluble micronutrient commonly found in the diet and plays an important role as a coenzyme factor in maintaining the body. Also, VitC is an important antioxidant that plays a vital role in immune function. It is well known that VitC is necessary for the biosynthesis of collagen. Interestingly, under certain conditions VitC can act as a pro-oxidant that acts to selectively suppress the growth of cancer cells. The antitumor effect of VitC was associated with reactive oxygen species (ROS) by administration of high-dose VitC in azoxymethane + dextran sodium sulfate-induced colorectal cancer in our previous study.) Additionally, the ROS effect caused by the administration of VitC to melanoma patients suggests an anticancer effect, indicating that ROS is crucial for the pathogenic function of high-dose VitC. Increased intracellular ROS formation has been linked to p53 expression, and VitC has been linked to p53, although it is unknown whether VitC affects endostatin.
In this study, the effects of high-dose VitC on p53, VEGF, and endostatin levels were examined in mice that had xenografts of Colon 26 murine colorectal cancer cells.
Effect of VC Administration on the Growth of Tumor Cells
In mice that received xenografts of Colon 26 tumor cells, tumors were seen. Administration of VitC slowed the growth of the tumor. When compared to the Colon 26 alone group, the tumor volume in the Colon 26 + VitC group was 28% lower (Fig. 1).
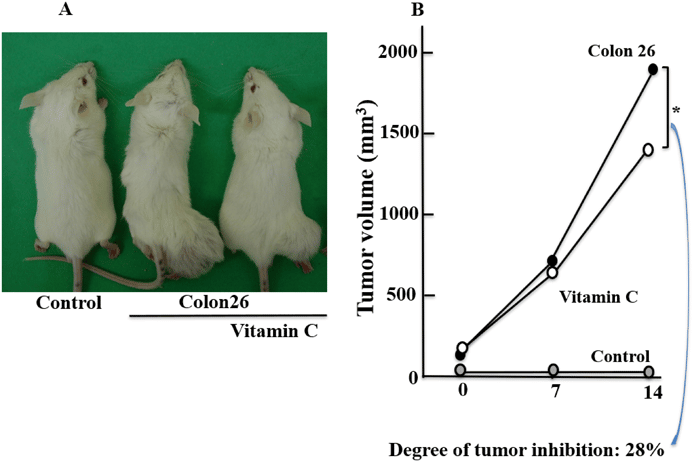
Figure 1 Effects of VitC Administration on Colon Growth
The tumor’s macroscopic appearance (A) and tumor volumes (B) are displayed. The data are shown as mean standard deviation (n = 6/group) * Tukey’s post-hoc test, p 0.05. (The online version has access to the color figure.)
The impact of VitC administration on p53, endostatin, HIF-1, and ROS levels
A gene called p53 promotes apoptosis in cancer cells. In comparison to the control group, the Colon 26 group had considerably higher levels of p53 in the tumor tissues of the lower limb. When compared to the Colon 26 group, the levels in the Colon 26 + VitC group were also significantly higher after receiving VitC treatment. We also evaluated plasma endostatin levels since p53-mediated tumor suppression is partly correlated with this protein. Similar to p53, endostatin levels were markedly higher in the Colon 26 group compared to the control group and even higher in the Colon 26 + VitC group (Figs. 2A, B). The Colon 26 group was shown to have significantly higher plasma and intratumoral ROS levels than the control group, but the Colon 26 + VitC group had significantly lower levels than the Colon 26 group. Similar to this, the HIF-1 levels in the tumor tissues of the lower limbs were considerably higher in the Colon 26 group than in the control group, but significantly lower in the Colon 26 + VitC group.
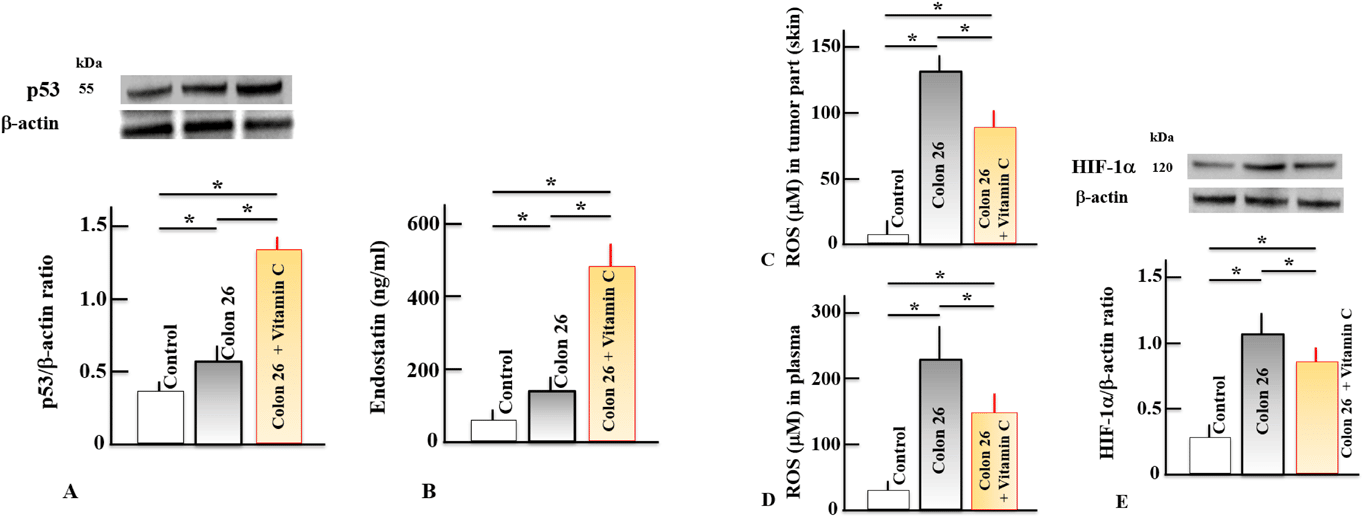
Figure 2(A-E) shows how the administration of VitC affected the levels of p53, ROS, and HIF-1 in the lower limb tumor tissues as well as endostatin and ROS in the plasma (D)
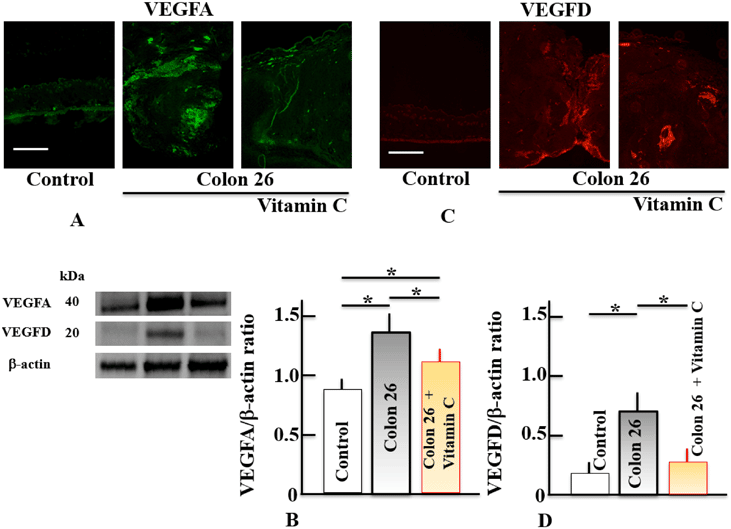
Figure 3 shows how VitC administration affected the levels of VEGFA (A, B) and VEGFD (C, D) in lower limb tumor tissues. Samples
DISCUSSION
The p53 gene stimulates transcription of the (II) collagen prolyl-4-hydroxylase gene, which is known to have anti-cancer properties. Strong endogenous angiogenesis inhibitors including endostatin and tumstatin, which are produced from collagen types XVIII and IV, are released extracellularly by this enzyme. These inhibitors reduce angiogenesis, which has anti-cancer properties. Tumor diameters were reduced in mice with xenografts of Colon 26 tumor cells following continuous VitC treatment for 14 days. Additionally, p53 expression levels, the levels of VEGFA and VEGFD were lowered, while and endostatin were elevated. Together, these findings imply that VitC might be a component of the p53/endostatin/VEGF circuit. Since the precise mechanism of action by which VitC raises endostatin levels is unknown, more investigation will be required in the future. Additionally, VitC has a role in collagen synthesis and maintenance and has the potential to directly influence collagen type XVIII without going through p53. Currently, this aspect is being looked into. Additionally, it has been noted that VitC blocks VEGF expression by way of HIF-1. Prolyl 4-hydroxylase (PHD), an oxygen-dependent enzyme, hydroxylates HIF-1, however in hypoxic conditions, HIF-1 stabilizes and generates transcriptional activators. After then, endothelial cells multiply, VEGF is released, and new blood vessels are created. ROS is also impacted by variations in oxygen content. ROS induce oxidative stress when the level of the defense system is exceeded. Additionally, it has been suggested that increased ROS levels aid in the stabilization of HIF during hypoxia and reoxygenation. Both ROS and HIF-1 levels were increased in mice with Colon 26 xenografts, but both were reduced when VitC was given. It has been proposed that the inhibition of angiogenesis by VitC is related to the inhibition of ROS by VitC. However, it is unclear whether this effect of VitC is affected by oxygen concentration, so further research is needed.
References
- The vascular endothelial growth factor (VEGF)/VEGF receptor system and its significance under healthy and pathological settings. 1) Takahashi H, Shibuya M. Clinical Science, 109, 227–241 (Lond (2005).
- Zhao Y and AA Adjei Beyond vascular endothelial growth factor: targeting angiogenesis in cancer treatment. Oncologist, age 20. (2015).
- Angiogenic cytokine: VEGF. Shintani S, Murohara T. J. Jpn. Coll. Angiol., 46, 289-295 (2006).
- Izawa N., Shitara K., Yonesaka K., Yamanaka T., Yoshino T., Sunakawa Y., Masuishi T., Denda T., Yamazaki K., Moriwaki T., Okuda H., Kondoh C., Nishina T., Makiyama A., Baba H., Yamaguchi H., Nakamura M., Hyo An exploratory study of the randomized phase 2 trial contrasting panitumumab and bevacizumab in conjunction with FOLFIRI found that the second-line treatment for KRAS exon2 wild-type metastatic colorectal cancer resulted in early tumor reduction and depth of response (WJOG6210G). Oncology Target, 15, 623-633 (2020).
- VEGF as a significant modulator of angiogenesis in cancer, Carmeliet P. 4-10 in Oncology, 69 (Suppl. 3). (2005).
- Forsythe JA, Jiang BH, Iyer NV, Agani F, Leung SW, Koos RD, and Semenza GL. Hypoxia-inducible factor 1 activates the transcription of the vascular endothelial growth factor gene. 16, 4604–4613 of Mol. Cell. Biol (1996).
- Sasaki T., Larsson H., Tisi D., Claesson-Welsh L., Hohenester E., and Timpl R. Different structure and binding characteristics, tissue distribution, and anti-angiogenic action distinguish endostatins generated from collagens XV and XVIII. 301, 1179–1190, J. Mol. Biol (2000).
- Folkman J. The antiangiogenic properties of endostatin and tumstatin play a role in p53’s ability to limit tumor growth. Pe35 in Sci. STKE, 2006 (2006).
- Ploegh HL, Olsen BR, Mothes W, Dreier L, Bryant RA. Collagen XVIII is converted into endostatin by secreted cathepsin L. EMBO J., 19, 1187-1194 (2000).
- Soond S.M., Kozhevnikova M.V., P.A. Townsend, and A.A. Zamyatnin Jr. networks that integrate the co-regulation of p53, microRNA, and cathepsin protease expression in cancer. Cancers (12, 3454) in Basel (2020).
- Holmannová D, Koláková M, and Krejsek No. 11 Vitamin C and its physiological function in relation to immune system elements. 58, 743–749 Vnitr. Lek (2012).
- Frei B, England L, and BN Ames Excellent antioxidant ascorbate is present in human blood plasma. Proc. National Academy of Sciences USA, 86, 6377-6381 (1989).
- Vitamin C and immunological function. Carr AC, Maggini S. 9, 1211 nutrients (2017).
- Li Y, Schellhorn HE. Novel therapeutic concepts for vitamin C: New advances. J. Nutr., 137, 2171-2184 (2007).
- Vitamin C and cancer revisited. Frei B, Lawson S. Proc. National Academy of Sciences USA, 105, 11037–11038 (2008).
- Kondo, K., Sano, R., Goto, K., and Ooi, K. Mice exposed to azoxymethane and dextran sodium sulfate develop colorectal cancer, which is lessened by the administration of large doses of vitamin C and irinotecan. 41, 1797-1803 Biol. Pharm. Bull (2018).
- Nakanishi K, Sato FE, Hiramoto K, and Ooi K. The invasion and growth of melanoma cells in the ovary of mice are inhibited by the administration of high doses of vitamin C. 44, 75-81 Biol. Pharm. Bull (2021).
- Ostrakhovitch EA and Cherian MG are on page 18. The function of p53 and reactive oxygen species in epithelial breast cancer cells’ sensitivity to copper and zinc-induced apoptosis. 10, 111–121 Apoptosis (2005).
- Lee MS, An SH, Kang JH, and Kim DH. When human colon cancer cells are treated with cisplatin, vitamin C induces an increase in apoptosis through the up-regulation of p53. 44, 211-216 BMB Rep. (2011).
- Shimizu T, Abu Lila AS, Nishio, Doi, Ando, M. Ukawa, Y. Ishima, and T. Ishida Modulation of antitumor immunity contributes to liposomal oxaliplatin’s improved therapeutic efficacy in mice models. 108, 1864-1869; Cancer Science (2017).
- Higashi T, Koshigoe T, Shimada Y, Hattori K, Takeuchi T, Arima H, Motoyama K, Onodera R Folate-appended -cyclodextrin is a promising in vitro and in vivo tumor targeting carrier for anticancer medications. 24, 724-733 Bioconjug. Chem (2013).
- Yokoyama, S., Hiramoto, K., Koyama, M., and K. Ooi In mice, indomethacin-induced small intestine damage is accompanied by skin disruption. 23, 659-663 in Exp. Dermatol (2014).
- Hiramoto K, Takahashi Y, Sugiyama D, Mafune E. The reduction of wrinkles brought on by skin dryness thanks to tranexamic acid. 80, 16–22 Biomed. Pharmacother (2016).
- Zhang Y, Zhao L, Wang J, Yu M, and Wang F. In lens epithelial cells, vitamin C reduces VEGF expression levels through pathways reliant and independent of hypoxia-inducible factor-1. 22. Mol. Med. Rep.
- Ke Q, Costa M. 25) HIF-1, or hypoxia-inducible factor (HIF-1). 70, 1469-1480 Mol. Pharmacol (2006).
- Rybnikova E., Vetrovoy O. An editorial on page 759 titled “Sex differences in neonatal mouse brain injury following hypoxia-ischemia and adaptaquin therapy” states that the neuroprotective activity of PHD inhibitors is primarily HIF-1-independent. 150, 645–647 J. Neurochem (2019).
- Chen R, Lai UH, Zhu, Singh, Ahmed, and Forsyth make up number 27. The function of hypoxia-inducible factors in reactive oxygen species production in the brain at various oxygen saturation levels. 132 Front. Cell. Dev. Biol (2018).