Role of Antisense Oligonucleotide Therapy in Pancreatic Cancer
Antisense oligonucleotides
Oligonucleotides are short sequences of nucleic acids either DNA or RNA that can bind complementary mRNA sequences in the nucleus and have the capability to inhibit the translation of mRNA in proteins.
Oligonucleotides were discovered about 20 years ago to inhibit the RNA and reduce the expression of specific proteins in the cell. They can be chemically modified and have different mechanisms of action in the cells. They have proved to be a promising therapy against neurological disorders and cancers.
The function of Antisense Oligonucleotides (ASOs)
Antisense oligonucleotide’s function is based on the structural complementarity to mRNA that is to be translated into a specific protein. They are usually used to target specific proteins. The proteins that are responsible for causing cancer in the cell are usually targeted.
ASOs can bind the specific mRNA as they have a sequence complementary to that mRNA. They are designed in the laboratory. After binding to mRNA, they will inhibit the transcription process, and no protein synthesis occurs. The low expression of that specific protein in the cell will affect many signaling pathways and the basic survival activities of that cell.
ASOs can also bind the specific protein instead of mRNA and degrade it decreasing its expression in the cell. They can also stop the transport of mRNA from the nucleus to the cytoplasm to inhibit the translation process. Translation depends on RNA transport from the nucleus to the cytoplasm and occurs in the cytoplasm. When mRNA is not transported, no translation of the target protein occurs (Rinaldi & Wood, 2018).

Pancreatic cancer
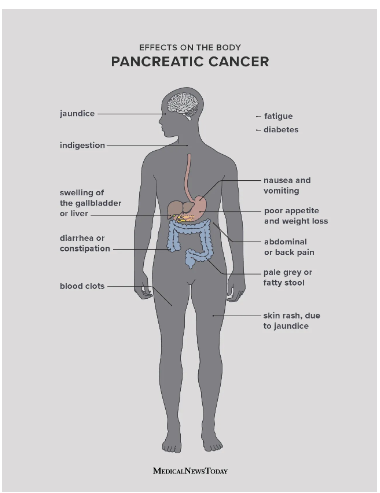
The abnormal mass of cells in the pancreatic tissue is called pancreatic carcinoma. The symptoms of this carcinoma include pain, jaundice, and weight loss. It is very difficult to diagnose it at the early stages of the disease. The Family Health history of the patient may affect the risk of disease in a family. Also, risk depends on the smoking habits of patients.
Risk factors
The risk factors for abnormal cell growth on the exocrine duct of the pancreas or the endocrine tissues of the pancreas include:
- Diabetes
- Smoking
- Family history of some genetic disorders like BRCA2 mutations in the family, FAMMM syndrome, and Lynch syndrome. These increase the risk of pancreatic cancer in patients.
- Obesity
- Patient’s Family history of pancreatic carcinoma
- Age is also a risk factor, as most people develop the disease after 65 years of age.
Mutations that cause Pancreatic cancer
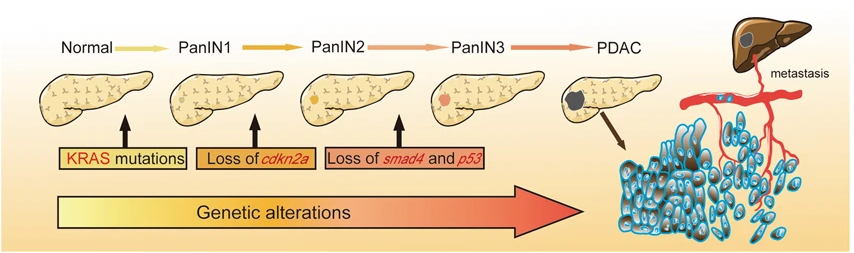
Pancreatic cancer is one of the most aggressive types of cancer. It cannot be diagnosed at the early stages and has a great mortality rate. Most patients with pancreatic carcinoma become resistant to available therapies like chemotherapy and radiotherapy. This cancer results from mutations in genes. These are the combination of mutations in oncogenes and tumor suppressor genes. The tumor-promoting genes are mutated in such a way that their function is stimulated while the function of tumor suppressors is inhibited by mutations in their genes (Hu et al., 2021).
By next-generation sequencing, scientists have recently identified many novel driver genes, and the mutations in these genes are playing role in the causation and progression of pancreatic cancer. KRAS is an oncogene, that exhibits gain-of-function mutation in pancreatic tumor cells. Similarly, the tumor suppressor genes such as P53. SMAD4, BRCA2, and CDNK2A have a loss of function mutations that can lead to the development of cancer in the pancreatic cells (Morioka et al., 2005).
Suppression of Pancreatic Cancer Oncogenesis by Antisense Oligonucleotides
Antisense oligonucleotides are proven to be a promising therapeutic strategy against different cancer types including pancreatic carcinoma. Oligonucleotides are sequences that target our genes of interest. They target one of the driver genes in pancreatic cancer cells as mentioned above. The mutated gene will be blocked in these cells either by oncogene or the tumor suppressor gene and will not be able to stimulate cells towards tumor phenotype anymore.
Antisense oligonucleotides to Gastrin
Gastrin acts as a stimulatory protein for the progression of pancreatic cancer. Experiments were performed both in-vitro and in-vivo on mice models to check if gastrin has a role in the progression of pancreatic cancer.
Anti-Gastrin oligonucleotides were given to the pancreatic cancer cell lines for 48 hours. Then the results were analyzed using a cell proliferation assay and were compared to the control. It was seen that the growth of pancreatic cancer cells was inhibited by 88% in the cells that were given anti-gastrin oligonucleotides as compared to the cells that were not given this therapy.
Anti-gastrin oligonucleotides have a sequence that is complementary to the sequence of gastrin mRNA. They bind it and block the translation process thus inhibiting the synthesis of gastrin. When the expression of gastrin decreases in the cells, tumor growth and progression stop (Smith et al., 1998).
Antisense Oligonucleotides to K-Ras
In-vitro experiments were performed on human pancreatic cell line Pc-2. These cells were treated with antisense oligonucleotides that have sequences complementary to the sequence of the point mutation in the K-Ras gene. These oligonucleotides bind and inhibit the synthesis of Ras protein, thus, decreasing its expression in the cells. These oligonucleotides were delivered into the cell by using liposomes. And afterward, results were analyzed using phase control microscopy, flow cytometry, and TUNNEL assay. It was observed that the process of apoptosis increased greatly in these cells as compared to the control cells.
Mutation in the Ras gene resulted in cell cycle arrest at the G1 phase for most of the pancreatic cancer cells. However, some cells also got arrested at the S-phase of the cell cycle. This also increased apoptosis and a large apoptosis peak was observed in the experimental cells (Yongxiang et al., 2014).
Antisense Oligonucleotides to KSR1 gene
KSR1 is a kinase suppressor of the Ras 1 gene. It is found experimentally in-vitro that the repression of this protein has an important link with the resistance against chemotherapeutic drugs in pancreatic cancer cells. To evaluate this, antisense oligonucleotides were designed for KSR1 and were delivered in the cells in-vitro. ELISA was performed and it was analyzed that if KSR1 is inhibited in the pancreatic cells, the resistance to the drugs may be reduced. It is not proven in clinical trials yet (Zhang et al., 2008).
Antisense Oligonucleotides for TGF-beta 2
TGF-beta is found to be highly upregulated in pancreatic cancer cells and stimulates the proliferation and metastasis of these cells. Targeting TGF-beta using antisense oligonucleotides can be an effective therapy to reduce tumor progression in pancreatic cells.
The in-vitro experiment was performed on the pancreatic cancer cell line, in which anti-TGF-beta2 oligonucleotides were delivered into the cells by liposomes. These cells were then tested for anti-tumor activity and compared with the control. It was observed in the cells receiving this therapy, that the proliferation was decreased by 76% when compared to other pancreatic cancer cells which were not given antisense oligonucleotides. So, ASO can be a promising therapy for treating pancreatic cancer in the near future.
Conclusion
Pancreatic cancer is one of the aggressive forms of cancer that can not be diagnosed at the early stages. Patients with pancreatic carcinoma develop resistance to the available treatments due to mutations in some driver genes like KRAS and TGF-beta genes. So, to decrease the resistance to therapies and to stop the progression of carcinoma, we need to target these driver genes. Many experiments are being performed in-vitro and in-vivo that use antisense oligonucleotides for these driver genes to inhibit them in the pancreatic cancer cells. These ASOs have proven to be effective in decreasing the progression of the disease. They will soon pass the clinical trials and pave the way through cancer therapeutics as an effective and safe therapy.
References
Hu, H. feng, Ye, Z., Qin, Y., Xu, X. wu, Yu, X. jun, Zhuo, Q. feng, & Ji, S. rong. (2021). Mutations in key driver genes of pancreatic cancer: molecularly targeted therapies and other clinical implications. Acta Pharmacologica Sinica, 42(11), 1725–1741. https://doi.org/10.1038/s41401-020-00584-2
Morioka, C. Y., Machado, M. C. C., Saito, S., Nakada, Y., Matheus, A. S., Jukemura, J., Bacchella, T., Takahara, T., & Watanabe, A. (2005). Suppression of invasion of a hamster pancreatic cancer cell line by antisense oligonucleotides mutation-matched to K-ras gene. In Vivo, 19(3), 535–538.
Rinaldi, C., & Wood, M. J. A. (2018). Antisense oligonucleotides: The next frontier for treatment of neurological disorders. Nature Reviews Neurology, 14(1), 9–22. https://doi.org/10.1038/nrneurol.2017.148
Smith, J. P., Verderame, M. F., & Zagon, I. S. (1998). Antisense oligonucleotides to gastrin inhibit growth of human pancreatic cancer. Cancer Letters, 135(1), 107–112. https://doi.org/10.1016/S0304-3835(98)00279-1
Yongxiang, W., Liang, G., & Qinshu, S. (2014). Apoptosis of human pancreatic carcinoma PC-2 cells by an antisense oligonucleotide specific to point mutated K-ras. Pathology and Oncology Research, 20(1), 81–85. https://doi.org/10.1007/s12253-013-9661-x
Zhang, J., Zafrullah, M., Yang, X., Yin, X., Zhang, Z., Fuks, Z., & Kolesnick, R. (2008). Downregulation of KSR1 in pancreatic cancer xenografts by antisense oligonucleotide correlates with tumor drug uptake. Cancer Biology and Therapy, 7(9), 1492–1497. https://doi.org/10.4161/cbt.7.9.6472